Periodic Table Questions
Directions
1. Using the Periodic Table, answer the following questions.
2. Be sure to use complete sentences.
Questions
1. ________________ was named after Ernest Rutherford.
2. ________________ was named after Niles Bohr.
3. ________________ was named after Marie Curie, the scientist who worked on radioactivity.
4. ________________ was named after Einstein.
5. ________________ was named after Alfred Nobel, who invented dynamite.
6. ________________ was named after Dmitry Mendeleev.
7. ________________ was named after Ernest Lawrence, who invented the cyclotron.
8. ________________, ________________ and _______________ were named for the planets.
9. ________________, ________________, ________________, and _______________ were named for four countries.
10. ________________ was named for a state.
11. ________________ was named for a city. (Hint: Itís in California!)
12. ________________ was named for a continent.
Homework: Look at the back cover of your textbook at the periodic table. List elements that you recognize and their uses. Example: Aluminum: Used for cans
Thursday, January 29, 2009
Tuesday, January 27, 2009
Atoms of Different Elements
Do all elements have the same atom? The answer is NO.
Today you will be investigating how the atom of 8 different elements looks.
Procedure
1. Fold a piece of paper into 8 sections
2. Go to Chemical Elements.com
3. Click on the first element "H" for Hydrogen
4. Scroll down to the Bohr diagram of Hydrogen
5. Draw the diagram in your first box on your paper. Title box as Hydrogen. Be sure to draw and label the nucleus, electons (with negative signs), protons (with positive signs), and neutrons (no charge).
6. Repeat steps 3-5 with the elements He, Li, Be, B, C, N, O
Questions: Please answer the following questions in complete sentences on the back of your paper.
1. What do you notice happens to the number of protons in each element as you move to the right on the periodic table?
2. What do you notice happens to the number of electrons in each element as you move to the right on the periodic table?
3. Is there a relationship between the number of protons and electrons found in an atom of a certain element? Why do you think this relationship exists?
4. If I told you that the element Silicon has 14 protons, how many electrons are in a Silicon atom?
5. Write an I learned statement about atoms.
Homework:None =)
Today you will be investigating how the atom of 8 different elements looks.
Procedure
1. Fold a piece of paper into 8 sections
2. Go to Chemical Elements.com
3. Click on the first element "H" for Hydrogen
4. Scroll down to the Bohr diagram of Hydrogen
5. Draw the diagram in your first box on your paper. Title box as Hydrogen. Be sure to draw and label the nucleus, electons (with negative signs), protons (with positive signs), and neutrons (no charge).
6. Repeat steps 3-5 with the elements He, Li, Be, B, C, N, O
Questions: Please answer the following questions in complete sentences on the back of your paper.
1. What do you notice happens to the number of protons in each element as you move to the right on the periodic table?
2. What do you notice happens to the number of electrons in each element as you move to the right on the periodic table?
3. Is there a relationship between the number of protons and electrons found in an atom of a certain element? Why do you think this relationship exists?
4. If I told you that the element Silicon has 14 protons, how many electrons are in a Silicon atom?
5. Write an I learned statement about atoms.
Homework:None =)
Monday, January 26, 2009
Atom Models
Thursday, January 22, 2009
Smelly Particles
Today in class we experienced how quickly the scent of popcorn can travel across the room. How does the scent get from the popcorn to your nose? What is the scent made out of?
Homework: What do particles have to do with smell? (2-3 sentences)
Homework: What do particles have to do with smell? (2-3 sentences)
Wednesday, January 21, 2009
Models
The word model has many meanings in the English Language. Models which are replicas of the real thing are useful in Science. They will be very useful when we are talking about atoms in our next unit on Chemistry. Today in  class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:
class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:
 class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:
class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:Thursday, January 15, 2009
Buoyancy!
Today in class we will be adding to our reasons of why things float in fluids (gases and liquids). The buoyant force (bf-our best friend) allows objects to float in fluids. The bf is an upward force that goes in the opposite direction of Weight.
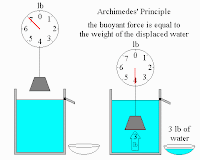
This diagram shows how an object has less weight when put in water. The amount of weight that it loses (the buoyant force) is equal to the weight of the water displaced by the object (Archimede's Priniciple).
Homework: Using the term buoyant force, explain how a boat floats (4-5 sentences).

This diagram shows how an object has less weight when put in water. The amount of weight that it loses (the buoyant force) is equal to the weight of the water displaced by the object (Archimede's Priniciple).
Homework: Using the term buoyant force, explain how a boat floats (4-5 sentences).
Tuesday, January 13, 2009
Regular Solids-Catch up Block Day
Today in class we will finish the Regular Solids Density lab and the second part of class will be a catch up day to finish work for the end of the semester.
Does the equation Volume = Length x Width x Height work to find the volume of all solids?
Does the equation Volume = Length x Width x Height work to find the volume of all solids?
Monday, January 12, 2009
Monday, January 12, 2009
Homework: Page 135 Do: Reading Check and practice problems 1-2 (bottom of page)
Thursday, January 8, 2009
Density on the Internet
Yesterday we practiced finding the mass and volume of irregular objects to calculate density.
Today you will be visiting a number of websites about density. Be sure to follow along on your worksheet to make sure that you are on the correct website to answer the questions.
Step One: Click Here to start with MASS (Continue to step two and three by clicking on the right arrow at the bottom of the page.
Step Two: Volume
Step Three: Density
Step Four: Click Here to see the dispacement simulation.
Step Five: Click Here to go to the "Why Do Things Float?" simulation.
Homework: None
Today you will be visiting a number of websites about density. Be sure to follow along on your worksheet to make sure that you are on the correct website to answer the questions.
Step One: Click Here to start with MASS (Continue to step two and three by clicking on the right arrow at the bottom of the page.
Step Two: Volume
Step Three: Density
Step Four: Click Here to see the dispacement simulation.
Step Five: Click Here to go to the "Why Do Things Float?" simulation.
Homework: None
Tuesday, January 6, 2009
Density Websites in Class
Today you will visit a few websites to practice finding the density of different substances. Please write answers to all questions on a piece of binder paper. You do not need to write the questions, but you do need to answer questions using complete sentence
1.Density Activity Scroll down and answer the questions in the boxes. Submit answers by clicking the submit button next to each answer. When you have correctly answered them, write the answers in complete sentences on binder paper.
2. Go to Explore Learning. Log in with your username and password. Click on the Density Gizmo. Explore the gizmo and answer the questions below the gizmo. Submit them and write answers on binder paper.
3. Answer the following questions after reading the M & M Article.
1) What is the main idea of this article?
2) How does this article relate to density?
3) How do you think this information could help people who buy candy? People who sell candy?
HOMEWORK: Read pg. 130-136 in textbook. Do p. 137, #1-5
1.Density Activity Scroll down and answer the questions in the boxes. Submit answers by clicking the submit button next to each answer. When you have correctly answered them, write the answers in complete sentences on binder paper.
2. Go to Explore Learning. Log in with your username and password. Click on the Density Gizmo. Explore the gizmo and answer the questions below the gizmo. Submit them and write answers on binder paper.
3. Answer the following questions after reading the M & M Article.
1) What is the main idea of this article?
2) How does this article relate to density?
3) How do you think this information could help people who buy candy? People who sell candy?
HOMEWORK: Read pg. 130-136 in textbook. Do p. 137, #1-5
Monday, January 5, 2009
Marble Density
Today in class we looked at a job aid to help us to find the density of a marble.
Homework: Using the density job aid, list the steps to find the density of a paper clip.
Homework: Using the density job aid, list the steps to find the density of a paper clip.
Subscribe to:
Comments (Atom)








