Extra Credit and all missing assignments are DUE on Friday December 18th.
Choose from the following two choices:
1. On page 446-447 pick from one of the following assignments: a) Something in the Air, b) What do you Mean by That? or c) Labeling Trans Fat
Use the following link for research: Real World Science Links
2. On page 442 do the Summarize assignment: Create your own lesson summary as you write a Newsletter. Follow the directions to complete the newsletter.
Friday, December 11, 2009
Thursday, December 3, 2009
Carbon Project
Carbon Project
Background
Carbon is arguably the most essential element on Earth.
Step One
Pick a topic for your project. Your topic must relate to why carbon is critical to life on Earth.
Possible Topics:
1. Carbon comes in many forms (graphite, diamond, charcoal)
2. Carbohydrates
3. Carbon fiber
4. Carbonation (in Soda!)
5. Chemical Bonding of Carbon
6. Photosynthesis
7. Carbon Cycle
8. Carbon Footprint
9. It’s All About Carbon
10. Carbon-Based Life
11. Carbon-Dating (Used to date archeological finds, fossils, etc.)
http://www.pbs.org/wgbh/nova/first/radiocarbon.htmlTo Research further you may also use nettrekker. which is an educational search engine.
Click on the following link: NETTREKKER
Login as Username: burnettstudent
password: student
Step Two
Research your chosen topic. Use the provided links. If you still need more information, use Google Search. Open a Pages blank document or MS Word file, title it: Carbon_mylastname_my firstname. Save it to your desktop (not to your digital locker, at least not yet). In the last five minutes of class, save your work in your digital locker. While researching, answer the following questions:
Topic #1: Carbon Comes In Many Forms
1. Pure carbon exists in how many different forms?
2. Can you find pure carbon in nature?n If yes, where?
What makes carbon look different in each form?
About Graphite
1. How is it made?
2. Is it a hard or soft material?
3. Describe 3 important uses of graphite.
About Diamonds
1. Where (which countries) is it found?
2. How far down in Earth are diamonds found?
3. How do humans "get" diamonds?
Topic #2: Carbohydrates
1. What is it?
2. What are carbohydrates used for?
3. Do carbohydrates come in different forms? If so, give examples.
4. How are carbohydrates made?
5. Why do humans like carbohydrates?
Topic #3: Carbon Fiber
1. What is carbon fiber?
2. How is carbon fiber made?
3. What is the history of carbon fiber?
4. What is carbon fiber used for?
Topic #4: Carbonation in Soda
1. What is it?
2. Why do we want carbonated soda?
3. Are we adding pure carbon when making carbonated soda?
Topic #5: Chemical Bonding
1. How many bonds does carbon form?
Topic #6: Photosynthesis
1. What is it?
2. What is carbon bonded to before the reaction?
3. What is carbon bonded to after the reaction?
Topic #7: Carbon Cycle
1. What is it?
2. What are the five main "parts" of Earth carbon moves (cycles) through?
3. What form does carbon take when it is in our atmosphere?
4. What form does carbon take when it is in our oceans?
5. How do plants and animals exchange (share) carbon? Explain how the process works.
Topic #8: Carbon Footprint
1. What is it?
2. How is the carbon footprint measured?
3. What is the history of the term?
Topic #9: It's All About Carbon (Overview)
1. Why is carbon the central element in life?
2. How is oil and coal made?
3. What can humans do to slow down global warming?
Topic #10: Carbon-based Life
1. How many atoms can carbon bond with?
2. Why do scientists think that other life forms will probably be made out of carbon?
3.
Topic #11: Carbon Dating
1. What is it?
2. What do we use carbon dating for?
3. How does carbon-dating work?
Step Three
Present your research, into one of the following ways:
- a Poster using Pages,
- a Cartoon using Comic Life,
- a research report using MS Word,
- a slide show using Keynote,
- a web using Inspiration 8
- a photo album using iPhoto
- a short video using iMovie
Thursday, November 19, 2009
Acid Base Indicator Project
Purpose: The purpose of this project is to communicate to the class your opinion as to which type of acid-base indicator is the most effective in identifying acids bases and neutral substances: litmus paper, cabbage juice, or pH paper.
Step 1: Research- Use the following websites to help you to make an educated decision as to which indicator is most effective:
Litmus Paper
What is litmus paper?
How litmus paper is made
Function of Litmus Paper
Cabbage Juice
Introduction to Red Cabbage Juice
More info on Cabbage juice
Step 2: Choose the type of indicator that you found was the best.
Step 3: Choose a media
Written Speech (3 paragraph minimum)
Poster using Pages
Keynote presentation
Step 4: Plan the Content
Content should include:
-What does the indicator look like?
-How does the indicator work?
-What information does the indicator give you?
-Reasons for why you think this is the best type of indicator.
-A counter-argument
Step 5: Save
Save the file as period_lastname_firstname_indicators
example: 1_foerder_diane_indicators
Drop it into my drop box
Step 1: Research- Use the following websites to help you to make an educated decision as to which indicator is most effective:
Litmus Paper
What is litmus paper?
How litmus paper is made
Function of Litmus Paper
Cabbage Juice
Introduction to Red Cabbage Juice
More info on Cabbage juice
pH Paper
What is pH?Step 2: Choose the type of indicator that you found was the best.
Step 3: Choose a media
Written Speech (3 paragraph minimum)
Poster using Pages
Keynote presentation
Step 4: Plan the Content
Content should include:
-What does the indicator look like?
-How does the indicator work?
-What information does the indicator give you?
-Reasons for why you think this is the best type of indicator.
-A counter-argument
Step 5: Save
Save the file as period_lastname_firstname_indicators
example: 1_foerder_diane_indicators
Drop it into my drop box
Monday, November 16, 2009
Tuesday, November 17th
Explore the kitchen for clues. For each clue write down what you think the clue is telling you. Use the clues to predict if each of the substances are acids, bases, or neutral. You can also try the bottle rockets with each substance.
Kitchen Chemistry
When you reach the Science Journal section make a data table to show your results:
Product Acid/Base/Neutral? Can be used
as Rocket
Fuel?
Mouthwash Base No
Vinegar
Pineapple Juice
Bottled Water
Orange Juice
Peppermint Extract
Lemon Juice
Stain Remover
Kitchen Chemistry
When you reach the Science Journal section make a data table to show your results:
Product Acid/Base/Neutral? Can be used
as Rocket
Fuel?
Mouthwash Base No
Vinegar
Pineapple Juice
Bottled Water
Orange Juice
Peppermint Extract
Lemon Juice
Stain Remover
Thursday, November 5, 2009
Thursday, November 5th
For today's assignment you will be balancing equations online. For each equation you balance on the computer, please copy and solve the equation on a piece of binder paper for your records. Click on the links below to start.
FunBrain Balancing Equations
Equations Fun
Balancing Equations Tutorial
Homework: Finish Balancing Equations Practice Worksheet
FunBrain Balancing Equations
Equations Fun
Balancing Equations Tutorial
Homework: Finish Balancing Equations Practice Worksheet
Wednesday, October 28, 2009
Wednesday, October 28th
Tuesday, October 27, 2009
Sunday, October 11, 2009
Monday October 12th: Building Molecular Models
Today we will learn about valence electrons and how atoms bond to form molecules in a compound.
Let's try out this virtual lab:
Molecular Models
Homework: Read pg. 218-228 Do pg. 228, #1-6
Let's try out this virtual lab:
Molecular Models
Homework: Read pg. 218-228 Do pg. 228, #1-6
Friday, October 9, 2009
Thursday, October 8, 2009
Thursday, October 8th
Start with the Compound Examples website.
1. Pick compounds that you are interested in.
2. In the first column write the name of the compound and its chemical formula. Also, describe the physical appearance of the compound.
3. In the 2nd column write the elements and how many atoms of each are in one molecule of this compound.
4. Look at the Wooden Periodic table for pictures of these elements and describe and draw the separate elements in the third column
Answer the following questions for HOMEWORK:
Questions: How are the elements similar to its compound? How are the elements different from its compound? In your examples are there more similarities or differences between the elements and the compounds?
Compound Examples
Wooden Periodic Table of Elements
1. Pick compounds that you are interested in.
2. In the first column write the name of the compound and its chemical formula. Also, describe the physical appearance of the compound.
3. In the 2nd column write the elements and how many atoms of each are in one molecule of this compound.
4. Look at the Wooden Periodic table for pictures of these elements and describe and draw the separate elements in the third column
Answer the following questions for HOMEWORK:
Questions: How are the elements similar to its compound? How are the elements different from its compound? In your examples are there more similarities or differences between the elements and the compounds?
Compound Examples
Wooden Periodic Table of Elements
Wednesday, October 7, 2009
Wednesday, October 7th
Fold a piece of binder paper into three columns. Label the 1st column ELEMENTS, the 2nd column COMPOUNDS, and the 3rd column MIXTURES. As you read websites 1-4, record information AND examples of each type under the appropriate columns. Use your notes to take the quiz .
1) Compounds and Mixtures Activity
2) Elements, Compounds, and Mixtures
3) Elements, Compounds, and Mixtures
4) Compounds
Mixtures, Elements, and Compounds Quiz#1
Element Math Game
1) Compounds and Mixtures Activity
2) Elements, Compounds, and Mixtures
3) Elements, Compounds, and Mixtures
4) Compounds
Mixtures, Elements, and Compounds Quiz#1
Element Math Game
Thursday, September 24, 2009
Thursday, September 24th
Here are the steps that you will follow for today's assignment.
- Open your digital locker by going up to "Go" on the finder window, "Connect to Server", type in server 10.200.4.11
- Username: First letter of first name followed by first five letters of last name and sometimes a number ex: My Name: Diane Foerder, Username: dfoerd
- Password: Last 4 digits of student ID number
- Open Student Files and Your digital locker
- Go into Student Files/ Assignments/ Foerder
- Drag Atoms file to your desktop, open and follow instructions
- When completed save to your digital locker in a Science Folder : period_last name_first_atoms
- To submit to my drop box open Student Files/Drop Boxes/drag or copy file into Foerder
Wednesday, September 9, 2009
9/9/09 Wednesday
Today we looked at how red dye moves in hot water vs. cold water.
Homework: Read pg. 255-259 Do: pg 260 #1-4
Homework: Read pg. 255-259 Do: pg 260 #1-4
Tuesday, May 26, 2009
Monday May 26th
Once you have finished the priority list on the board:
1. Inner Foldable
2. Outer Foldable
3. Inner Outer Planets Venn Diagram
4. Powers of Ten pictures
5. Guide to the Galaxy
Go on to the Mars Mission site to interact with different mars rover activities
CLICK HERE
Homework: None
1. Inner Foldable
2. Outer Foldable
3. Inner Outer Planets Venn Diagram
4. Powers of Ten pictures
5. Guide to the Galaxy
Go on to the Mars Mission site to interact with different mars rover activities
CLICK HERE
Homework: None
Thursday, May 21, 2009
Tuesday, May 12, 2009
May 12th/13th Tues./Wed.
1, 4, 5th period
Today we will take a virtual tour of Saturn's system.
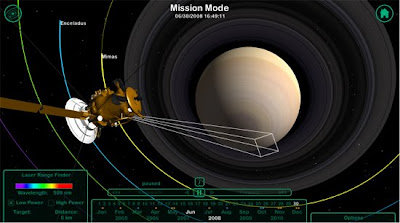
Click on the picture above to start your tour! Explore all the different places the simulation can take you?
On binder paper:
1. List 10 facts that you learn about Saturn or the Cassini Mission through exploring the simulation.
2. Choice: Choose from the following two essay questions to answer in 3-4 paragraphs
a. What do you think is the purpose of missions such as Cassini? NASA spends billions of dollars to send missions like Cassini into Space. Do you think it is worth it? (persuasive essay)
b. Write a guide that would go with the Cassini simulation, leading a student through important parts of the tour. Include questions that a student could answer as they follow your guide.
Ex. 1. Click on "Moon Mode" at the bottom of the screen.
2. Click on Titan
a. Describe Titan's atmosphere
Today we will take a virtual tour of Saturn's system.

Click on the picture above to start your tour! Explore all the different places the simulation can take you?
On binder paper:
1. List 10 facts that you learn about Saturn or the Cassini Mission through exploring the simulation.
2. Choice: Choose from the following two essay questions to answer in 3-4 paragraphs
a. What do you think is the purpose of missions such as Cassini? NASA spends billions of dollars to send missions like Cassini into Space. Do you think it is worth it? (persuasive essay)
b. Write a guide that would go with the Cassini simulation, leading a student through important parts of the tour. Include questions that a student could answer as they follow your guide.
Ex. 1. Click on "Moon Mode" at the bottom of the screen.
2. Click on Titan
a. Describe Titan's atmosphere
Tuesday, April 21, 2009
Tues./Wed. April 21/22
Today we will explore chemical reactions and watch some demonstrations. The five pieces of evidence that prove that a chemical reactions took place are:
Homework: None
- Bubbles form- a gas is given off
- A color change occurs
- A precipitate is formed-a solid substance is formed from two or more liquids
- Energy change
- A new substance is formed (all chemical reactions need this piece of evidence)
Homework: None
Monday, April 20, 2009
Monday April 20th
Today in class we learned about the differences between chemical and physical changes.
HW for periods 1, 2, 5, 6: p. 344 # 1-6
HW for period 4: p. 318 # 1-6
HW for periods 1, 2, 5, 6: p. 344 # 1-6
HW for period 4: p. 318 # 1-6
Thursday, April 9, 2009
Thursday, April 9th
Use the following links to complete the Matter Webquest in your assignments folder.
Before Starting: Fun Brain
Homework: Complete extra credit p 63-79 in workbook and finish cartoon if you didn't turn it in today
Before Starting: Fun Brain
Homework: Complete extra credit p 63-79 in workbook and finish cartoon if you didn't turn it in today
Thursday, April 2, 2009
Thursday, April 2nd
Today we started working on the cartoon. Students had very creative ideas as to what to use as their main character.
Homework: Finish cartoon rough draft
Homework: Finish cartoon rough draft
Tuesday, March 31, 2009
Tues./Wed. March 31-April 1
Today in class we finished up the distillation lab and wrote conclusions about what we learned. We also were presented with our next project : Changes in Matter Cartoon.
Homework: Choose a character for your cartoon. Make sure the character can turn from a solid to a liquid, and then a liquid to a gas. (ex. ice cube, popsicle, snow man, ice cream, chocolate) Draw or print from a computer the solid, liquid, and gas form of your character.
Homework: Choose a character for your cartoon. Make sure the character can turn from a solid to a liquid, and then a liquid to a gas. (ex. ice cube, popsicle, snow man, ice cream, chocolate) Draw or print from a computer the solid, liquid, and gas form of your character.
Monday, March 30, 2009
Monday March 30th
Today we learned the wonders of distillation. Here is a picture of the results from our distillation of grape juice. Many students were surprised when I poured clear water from the bowl in the pot.
 Look how dark the liquid that was left at the bottom of the pot is. Is this liquid the solvent or the solute?
Look how dark the liquid that was left at the bottom of the pot is. Is this liquid the solvent or the solute?
Homework: Part 1 and Lesson Review from the following worksheet.

 Look how dark the liquid that was left at the bottom of the pot is. Is this liquid the solvent or the solute?
Look how dark the liquid that was left at the bottom of the pot is. Is this liquid the solvent or the solute?Homework: Part 1 and Lesson Review from the following worksheet.

Tuesday, March 24, 2009
Tues./Wed. March 24/25
Monday, March 23, 2009
Monday, March 23rd
Friday, March 13, 2009
Friday the 13th! (March)
Remember, today in class you need to drop your brochure document into my drop box on the server by the end of class.
For those who finish early-you have two choices:
1. Make your own crossword puzzle using information from your brochure. Use the puzzlemaker on the discovery site to make a puzzle with at least 10 words and clues.
Crossword Puzzlemaker
Print out the puzzle for your friends to try!
2. Write a test on pages or appleworks about metals, nonmetals, metalloids, and noble gases.
Your test needs: 2 true/false questions, 4 multiple choice questions, 4 fill in the blank statements, and 1 essay.
I will use any terrific questions in our real test. Wouldn't that be nice to take a test with questions that you wrote?
Homework: Have a wonderful weekend!
For those who finish early-you have two choices:
1. Make your own crossword puzzle using information from your brochure. Use the puzzlemaker on the discovery site to make a puzzle with at least 10 words and clues.
Crossword Puzzlemaker
Print out the puzzle for your friends to try!
2. Write a test on pages or appleworks about metals, nonmetals, metalloids, and noble gases.
Your test needs: 2 true/false questions, 4 multiple choice questions, 4 fill in the blank statements, and 1 essay.
I will use any terrific questions in our real test. Wouldn't that be nice to take a test with questions that you wrote?
Homework: Have a wonderful weekend!
Thursday, March 12, 2009
Thursday, March 12th
Today in class we continued working on our brochure for the periodic table. It is due tomorrow by the end of the period! Be sure to check the board for questions you should ask yourself before submitting the brochure to my drop box:
1. Did you include the properties of each group?
2. Did you describe where to find each group on the periodic table?
3. Did you include two examples of elements and their uses for each group?
4. Did you include at least one picture per group?
Homework: pg. 246-247 Answer questions: 1, 3, 8, 12, 17, 18, 23-26
1. Did you include the properties of each group?
2. Did you describe where to find each group on the periodic table?
3. Did you include two examples of elements and their uses for each group?
4. Did you include at least one picture per group?
Homework: pg. 246-247 Answer questions: 1, 3, 8, 12, 17, 18, 23-26
Tuesday, March 3, 2009
Tuesday/Wednesday March 3rd/4th
Today in class you will be designing a brochure for the periodic table. A template for the brochure can be found in my assignments folder in Student Files.
You need to include the following:
A section for:
metals
non metals
metalloids
noble gases
Each section should include:
1. A description of the properties of the elements in that group
2. A description of where the elements can be found on the periodic table
3. Examples of elements in the group with common uses
4. Pictures
Web Sites for research:
Metals, Non metals, and Metalloids
More Metals, Non metals, and Metalloids
Periodic Table with Descriptions of Groups
Periodic Table Web sites:
Periodic Table Collector -(really cool website)
Dynamic Periodic Table
Periodic Table
Another Periodic Table
Periodic Table and Minerals
Homework: Which group (metals, non metals, metalloids, noble gases) is most essential for life? Use information you gathered from your research to support your decision. (4-5 sentences)
You need to include the following:
A section for:
metals
non metals
metalloids
noble gases
Each section should include:
1. A description of the properties of the elements in that group
2. A description of where the elements can be found on the periodic table
3. Examples of elements in the group with common uses
4. Pictures
Web Sites for research:
Metals, Non metals, and Metalloids
More Metals, Non metals, and Metalloids
Periodic Table with Descriptions of Groups
Periodic Table Web sites:
Periodic Table Collector -(really cool website)
Dynamic Periodic Table
Periodic Table
Another Periodic Table
Periodic Table and Minerals
Homework: Which group (metals, non metals, metalloids, noble gases) is most essential for life? Use information you gathered from your research to support your decision. (4-5 sentences)
Monday, March 2, 2009
Monday March 2nd
Today we tested different substances to see if they were metals, non metals, or both. The main properties we were testing for were luster, malleability, and conductivity. There were a few surprises so far, like shiny wires from inside a tv did not conduct electricity and a piece of graphite for a mechanical pencil did conduct electricity.
Homework: None
Homework: None
Thursday, February 26, 2009
Metals and Nonmetals
Wednesday, February 25, 2009
Tuesday/Wednesday
Students learned how to predict and then build models of common molecules such as carbon dioxide, methane, ethane
Homework: None
Homework: None
Tuesday, February 24, 2009
Monday
We started our molecular models using marshmallows and pasta bonds.
Homework: What is the difference between molecules and compounds? (2-3 sentences)
Homework: What is the difference between molecules and compounds? (2-3 sentences)
Thursday, February 19, 2009
Teachers in Training
Today students played teacher and taught each other about ionic and covalent bonds. We also learned more about why certain elements bond better with each other= valence electrons! The valence electrons of atoms that bond should add up to 8. Here is a chart showing how elements in the same column have the same number of valence electrons, or electrons on the very edge of the atom that are involved in chemical bonds:
 For Example: Lithium (1 valence e-) bonds with Fluorine (7 valence e-) because they add up to 8 to make the bond noble.
For Example: Lithium (1 valence e-) bonds with Fluorine (7 valence e-) because they add up to 8 to make the bond noble.
Homework: None
 For Example: Lithium (1 valence e-) bonds with Fluorine (7 valence e-) because they add up to 8 to make the bond noble.
For Example: Lithium (1 valence e-) bonds with Fluorine (7 valence e-) because they add up to 8 to make the bond noble.Homework: None
Wednesday, February 18, 2009
Ionic Vs. Covalent Bonding
Today in class you watched an animation of how ionic bonds occur and how covalent bonds occur. Here is a link to that animation and a link to the differences between the two types of bonds.
Bonding Animation
Ionic vs. Covalent Bonding
Homework: In class you were assigned covalent or ionic bonds. Your assignment is to prepare a presentation (3-4 min) with at least 1 visual (picture, drawing, pic from internet) about your assigned type of bond to share with your partner. It is your responsibility to come prepared to teach your partner, do not let them down!
Bonding Animation
Ionic vs. Covalent Bonding
Homework: In class you were assigned covalent or ionic bonds. Your assignment is to prepare a presentation (3-4 min) with at least 1 visual (picture, drawing, pic from internet) about your assigned type of bond to share with your partner. It is your responsibility to come prepared to teach your partner, do not let them down!
Tuesday, February 10, 2009
Compounds!
Pick compounds from these links to write the chemical formulas.
You can also use Google to search for common substances that are not found on these websites.
Example: Compound: Salt Chemical Formula: NaCl Recipe: 1 Sodium and 1 Chlorine
Homework: Find the definition of a mixture in your book. How is a mixture different than a compound? (3-4 sentences)
Homework: Find the definition of a mixture in your book. How is a mixture different than a compound? (3-4 sentences)
Monday, February 9, 2009
Persuasive Essay
Today we worked on our persuasive essay answering the question: What is your opinion on using science and technology in war?
Outline:
Paragraph 1: Introduction-Take a stance and briefly list three reasons to support your opinion.
Paragraph 2: Reason 1 with three details to support it
Paragraph 3: Reason 2 with three details to support it
Paragraph 4: Reason 3 with three details to support it
Paragraph 5: Counter-argument: include what the other side would say about technology and science in war, but then refute it with evidence from your own stance.
Paragraph 6: Conclusion
Thursday, February 5, 2009
Technology of war
After an atomic bomb presentation, students will write a persuasive essay that either defends the use of technology and science in war, or goes against the use of these technologies during war.
Websites for further research:
How Stuff Works: Technology of War
Atomic Archive.com
Remember a persuasive essay needs the following:
1. Three developed reasons with 3 details of why you are for or against technology and science in war.
2. A counterargument- What would the other side say?
3. A clear introduction and conclusion
4. Watch for grammar and spelling
Homework: brainstorm ideas for persuasive essay
Websites for further research:
How Stuff Works: Technology of War
Atomic Archive.com
Remember a persuasive essay needs the following:
1. Three developed reasons with 3 details of why you are for or against technology and science in war.
2. A counterargument- What would the other side say?
3. A clear introduction and conclusion
4. Watch for grammar and spelling
Homework: brainstorm ideas for persuasive essay
Tuesday, February 3, 2009
Isotopes and Ions Continued plus the Atomic Bomb
What is an isotope? It is an atom that has the correct number of protons, but the number of neutrons has changed. Carbon-14 is an example of an isotope that has 2 extra neutrons in its nucleus. Carbon-14 is used to determine how long ago different dead organisms existed.

What is an ion? It is an atom that has lost or gained electrons. Ions are charged atoms. Why don't neutrons affect the charge of an atom? Think about the charge that a neutron has.
Homework: None

What is an ion? It is an atom that has lost or gained electrons. Ions are charged atoms. Why don't neutrons affect the charge of an atom? Think about the charge that a neutron has.
Homework: None
Monday, February 2, 2009
Atom Identity, Ions, and Isotopes
Today in class we started working on the Element Builder simulation on www.explorelearning.com.
Homework: Pg. 205 Do #1-5
Homework: Pg. 205 Do #1-5
Thursday, January 29, 2009
Periodic Table Questions
Periodic Table Questions
Directions
1. Using the Periodic Table, answer the following questions.
2. Be sure to use complete sentences.
Questions
1. ________________ was named after Ernest Rutherford.
2. ________________ was named after Niles Bohr.
3. ________________ was named after Marie Curie, the scientist who worked on radioactivity.
4. ________________ was named after Einstein.
5. ________________ was named after Alfred Nobel, who invented dynamite.
6. ________________ was named after Dmitry Mendeleev.
7. ________________ was named after Ernest Lawrence, who invented the cyclotron.
8. ________________, ________________ and _______________ were named for the planets.
9. ________________, ________________, ________________, and _______________ were named for four countries.
10. ________________ was named for a state.
11. ________________ was named for a city. (Hint: Itís in California!)
12. ________________ was named for a continent.
Homework: Look at the back cover of your textbook at the periodic table. List elements that you recognize and their uses. Example: Aluminum: Used for cans
Directions
1. Using the Periodic Table, answer the following questions.
2. Be sure to use complete sentences.
Questions
1. ________________ was named after Ernest Rutherford.
2. ________________ was named after Niles Bohr.
3. ________________ was named after Marie Curie, the scientist who worked on radioactivity.
4. ________________ was named after Einstein.
5. ________________ was named after Alfred Nobel, who invented dynamite.
6. ________________ was named after Dmitry Mendeleev.
7. ________________ was named after Ernest Lawrence, who invented the cyclotron.
8. ________________, ________________ and _______________ were named for the planets.
9. ________________, ________________, ________________, and _______________ were named for four countries.
10. ________________ was named for a state.
11. ________________ was named for a city. (Hint: Itís in California!)
12. ________________ was named for a continent.
Homework: Look at the back cover of your textbook at the periodic table. List elements that you recognize and their uses. Example: Aluminum: Used for cans
Tuesday, January 27, 2009
Atoms of Different Elements
Do all elements have the same atom? The answer is NO.
Today you will be investigating how the atom of 8 different elements looks.
Procedure
1. Fold a piece of paper into 8 sections
2. Go to Chemical Elements.com
3. Click on the first element "H" for Hydrogen
4. Scroll down to the Bohr diagram of Hydrogen
5. Draw the diagram in your first box on your paper. Title box as Hydrogen. Be sure to draw and label the nucleus, electons (with negative signs), protons (with positive signs), and neutrons (no charge).
6. Repeat steps 3-5 with the elements He, Li, Be, B, C, N, O
Questions: Please answer the following questions in complete sentences on the back of your paper.
1. What do you notice happens to the number of protons in each element as you move to the right on the periodic table?
2. What do you notice happens to the number of electrons in each element as you move to the right on the periodic table?
3. Is there a relationship between the number of protons and electrons found in an atom of a certain element? Why do you think this relationship exists?
4. If I told you that the element Silicon has 14 protons, how many electrons are in a Silicon atom?
5. Write an I learned statement about atoms.
Homework:None =)
Today you will be investigating how the atom of 8 different elements looks.
Procedure
1. Fold a piece of paper into 8 sections
2. Go to Chemical Elements.com
3. Click on the first element "H" for Hydrogen
4. Scroll down to the Bohr diagram of Hydrogen
5. Draw the diagram in your first box on your paper. Title box as Hydrogen. Be sure to draw and label the nucleus, electons (with negative signs), protons (with positive signs), and neutrons (no charge).
6. Repeat steps 3-5 with the elements He, Li, Be, B, C, N, O
Questions: Please answer the following questions in complete sentences on the back of your paper.
1. What do you notice happens to the number of protons in each element as you move to the right on the periodic table?
2. What do you notice happens to the number of electrons in each element as you move to the right on the periodic table?
3. Is there a relationship between the number of protons and electrons found in an atom of a certain element? Why do you think this relationship exists?
4. If I told you that the element Silicon has 14 protons, how many electrons are in a Silicon atom?
5. Write an I learned statement about atoms.
Homework:None =)
Monday, January 26, 2009
Atom Models
Thursday, January 22, 2009
Smelly Particles
Today in class we experienced how quickly the scent of popcorn can travel across the room. How does the scent get from the popcorn to your nose? What is the scent made out of?
Homework: What do particles have to do with smell? (2-3 sentences)
Homework: What do particles have to do with smell? (2-3 sentences)
Wednesday, January 21, 2009
Models
The word model has many meanings in the English Language. Models which are replicas of the real thing are useful in Science. They will be very useful when we are talking about atoms in our next unit on Chemistry. Today in  class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:
class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:
 class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:
class we learned that there are good models and bad models. What makes something a good model? Let's looks at these two models of a car:Thursday, January 15, 2009
Buoyancy!
Today in class we will be adding to our reasons of why things float in fluids (gases and liquids). The buoyant force (bf-our best friend) allows objects to float in fluids. The bf is an upward force that goes in the opposite direction of Weight.
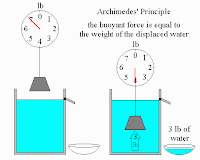
This diagram shows how an object has less weight when put in water. The amount of weight that it loses (the buoyant force) is equal to the weight of the water displaced by the object (Archimede's Priniciple).
Homework: Using the term buoyant force, explain how a boat floats (4-5 sentences).

This diagram shows how an object has less weight when put in water. The amount of weight that it loses (the buoyant force) is equal to the weight of the water displaced by the object (Archimede's Priniciple).
Homework: Using the term buoyant force, explain how a boat floats (4-5 sentences).
Tuesday, January 13, 2009
Regular Solids-Catch up Block Day
Today in class we will finish the Regular Solids Density lab and the second part of class will be a catch up day to finish work for the end of the semester.
Does the equation Volume = Length x Width x Height work to find the volume of all solids?
Does the equation Volume = Length x Width x Height work to find the volume of all solids?
Monday, January 12, 2009
Monday, January 12, 2009
Homework: Page 135 Do: Reading Check and practice problems 1-2 (bottom of page)
Thursday, January 8, 2009
Density on the Internet
Yesterday we practiced finding the mass and volume of irregular objects to calculate density.
Today you will be visiting a number of websites about density. Be sure to follow along on your worksheet to make sure that you are on the correct website to answer the questions.
Step One: Click Here to start with MASS (Continue to step two and three by clicking on the right arrow at the bottom of the page.
Step Two: Volume
Step Three: Density
Step Four: Click Here to see the dispacement simulation.
Step Five: Click Here to go to the "Why Do Things Float?" simulation.
Homework: None
Today you will be visiting a number of websites about density. Be sure to follow along on your worksheet to make sure that you are on the correct website to answer the questions.
Step One: Click Here to start with MASS (Continue to step two and three by clicking on the right arrow at the bottom of the page.
Step Two: Volume
Step Three: Density
Step Four: Click Here to see the dispacement simulation.
Step Five: Click Here to go to the "Why Do Things Float?" simulation.
Homework: None
Tuesday, January 6, 2009
Density Websites in Class
Today you will visit a few websites to practice finding the density of different substances. Please write answers to all questions on a piece of binder paper. You do not need to write the questions, but you do need to answer questions using complete sentence
1.Density Activity Scroll down and answer the questions in the boxes. Submit answers by clicking the submit button next to each answer. When you have correctly answered them, write the answers in complete sentences on binder paper.
2. Go to Explore Learning. Log in with your username and password. Click on the Density Gizmo. Explore the gizmo and answer the questions below the gizmo. Submit them and write answers on binder paper.
3. Answer the following questions after reading the M & M Article.
1) What is the main idea of this article?
2) How does this article relate to density?
3) How do you think this information could help people who buy candy? People who sell candy?
HOMEWORK: Read pg. 130-136 in textbook. Do p. 137, #1-5
1.Density Activity Scroll down and answer the questions in the boxes. Submit answers by clicking the submit button next to each answer. When you have correctly answered them, write the answers in complete sentences on binder paper.
2. Go to Explore Learning. Log in with your username and password. Click on the Density Gizmo. Explore the gizmo and answer the questions below the gizmo. Submit them and write answers on binder paper.
3. Answer the following questions after reading the M & M Article.
1) What is the main idea of this article?
2) How does this article relate to density?
3) How do you think this information could help people who buy candy? People who sell candy?
HOMEWORK: Read pg. 130-136 in textbook. Do p. 137, #1-5
Monday, January 5, 2009
Marble Density
Today in class we looked at a job aid to help us to find the density of a marble.
Homework: Using the density job aid, list the steps to find the density of a paper clip.
Homework: Using the density job aid, list the steps to find the density of a paper clip.
Subscribe to:
Posts (Atom)












